Aspergillus
Aspergillus is a very large genus containing about 250 species, which are currently classified into seven subgenera that are in turn subdivided into several sections comprised of related species (Raper and Fennell 1965, Gams et al. 1985, Geiser et al. 2007).
Traditionally, clinical microbiology laboratories have relied heavily on morphology-based identification methods to differentiate Aspergillus species. However many species, especially members of the section Fumigati have overlapping morphological characteristics, which has allowed several genetically distinct species to be misidentified (Balajee et al. 2005, 2007). This has led to the clustering of species with overlapping morphologies into “species complexes”, so that laboratories may report more accurately morphology-based identifications.
-
Laboratory identification
Identification of clinical isolates of Aspergillus to species level may be important given that different species have variable susceptibilities to multiple antifungal drugs. For example, in vitro and in vivo studies have demonstrated that A. terreus isolates are largely resistant to the antifungal drug amphotericin B, A. ustus isolates appear to be refractory to azoles, and A. lentulus and A. alliaceus have low in vitro susceptibilities to a wide range of antifungals including amphotericin B, azoles, and echinocandins (Balajee et al. 2005, 2007).
Molecular identification:
Recommended barcoding gene: β-tubulin. General criteria for identification were outlined by Balajee et al. (2007). Phylogenetic relationships of the entire genus were presented by Wang et al. (1999) and Peterson (2000, 2008).MALDI-TOF MS:
A comprehensive ‘in-house’ database of reference spectra allows accurate identification of species of Aspergillus even within complexes e.g. A. fumigatus sensu stricto and A. lentulus (Lau et al. 2013, Sleiman et al. 2015).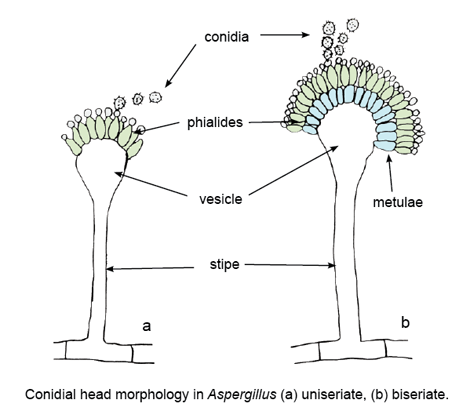
Morphological description:
Colonies are usually fast growing, white, yellow, yellow-brown, brown to black or shades of green, mostly consisting of a dense felt of erect conidiophores. Conidiophores terminate in a vesicle covered with either a single palisade-like layer of phialides (uniseriate) or a layer of subtending cells (metulae) which bear small whorls of phialides (the biseriate structure). The vesicle, phialides, metulae (if present) and conidia form the conidial head. Conidia are one-celled, smooth or rough-walled, hyaline or pigmented, are produced in long dry chains which may be divergent (radiate) or aggregated in compact columns (columnar). Some species may produce Hülle cells or sclerotia.For morphological identification, isolates are usually inoculated at three points on Czapek Dox agar and 2% malt extract agar and incubated at 25C. Most species sporulate within 7 days. Descriptions are primarily based on colony pigmentation and morphology of the conidial head. Microscopic mounts are best made using cellotape flag or slide culture preparations mounted in lactophenol cotton blue. A drop of alcohol is usually needed to remove bubbles and excess conidia.
Key features:
Hyaline hyphomycete showing distinctive conidial heads with flask-shaped phialides arranged in whorls on a vesicle.References:
Raper and Fennell (1965), Domsch et al. (1980), McGinnis (1980), Onions et al. (1981), Samson and Pitt (1990, 2000), Samson et al. (1995), Samson (1979), Vanden Bossche et al. (1988), Klich (2002), Steinbach et al. (2005), Samson et al. (2011a, 2014), de Hoog et al. (2000, 2015).
Species descriptions
-
Aspergillus felis
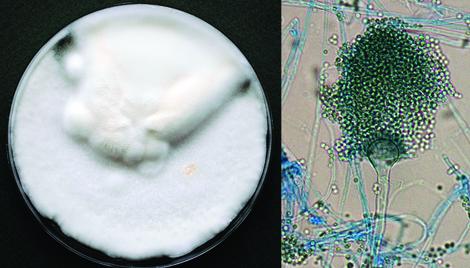
Culture and conidial head of Aspergillus felis.
Aspergillus felis is a member of the Aspergillus viridinutans complex within section Fumigati (Novakova et al., 2013) and has been reported as a causative agent of invasive aspergillosis and rhinosinusitis in humans, dogs and cats. Disease in all host species is often refractory to aggressive antifungal therapeutic regimens.
RG-1 organism.
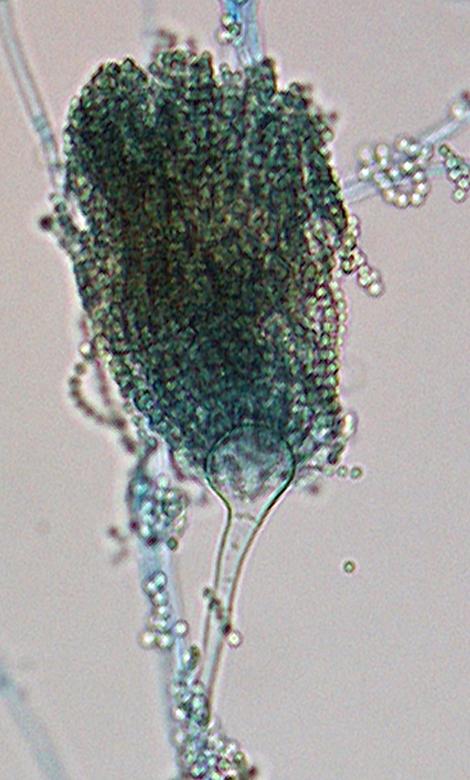
Aspergillus felis conidial head morphology.
Morphological description:
Colonies of A. felis are suede-like to floccose, white with interspersed grey green patches of conidia (conidiation is slow to poor). Conidial heads of A. felis are short, columnar and uniseriate. Conidiophore stipes are smooth-walled and vesicles are usually subglobose in shape. Conidia globose (2-3 µm in diameter), smooth to finely roughened.Molecular identification:
A. felis can be distinguished from other members of the section Fumigati by sequence analysis of β-tubulin, calmodulin and actin genes (Barrs et al. 2013). ITS sequencing is not recommended.Comment:
A. felis is phenotypically similar to Aspergillus viridinutans, but differs by its ability to grow at 45°C. This species is phylogenetically related to A. aureoluteus and A. udagawae and differs from A. aureoluteus by having a heterothallic mode of reproduction.Antifungal susceptibility: Aspergillus felis (Australian national data); MIC µg/mL, *MEC µg/mL. No ≤0.016 0.03 0.06 0.125 0.25 0.5 1 2 4 ≥8 AmB 21 1 11 3 5 1 ISAV 3 3 VORI 21 1 3 3 6 8 POSA 21 4 3 2 1 9 2 ITRA 21 1 2 2 4 2 9 1 ANID* 5 4 1 MICA* 5 3 2 -
Aspergillus fischeri
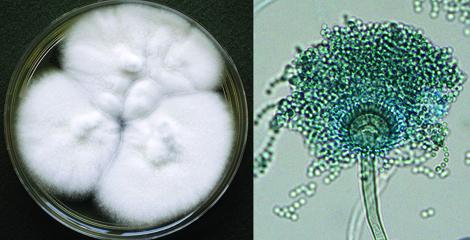
Aspergillus fischeri culture and conidial head.
Synonymy: Neosartorya fischeri
Aspergillus fischeri is a member of the A. fumigatus complex and is mostly found in canned foodstuffs and is now documented as a causative agent of invasive aspergillosis in immunosuppressed patients.
RG-1 organism.
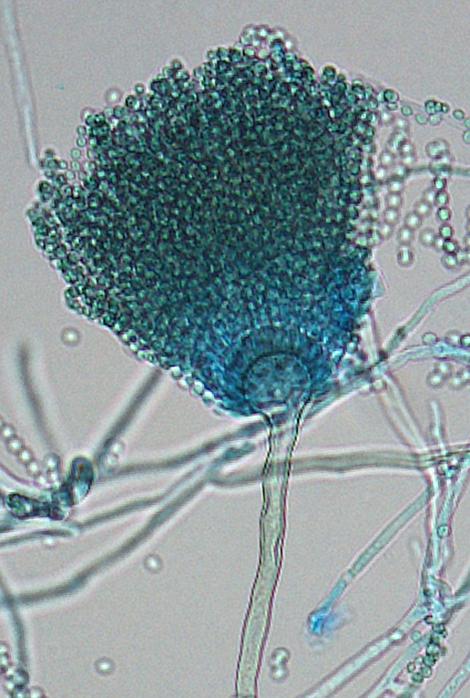
Aspergillus fischeri conidial head morphology.
Morphological description:
Colonies of A. fischeri are suede-like to floccose, white to pale yellow with slow to poor conidiation. Conidial heads are short, columnar and uniseriate. Conidiophore stipes are smooth-walled and vesicles are usually subglobose to flask-shaped. Conidia globose to subglobose (2-2.5 µm in diameter), smooth to finely roughened. Good growth at 37C.Molecular identification:
A. fischeri can be distinguished from other members of the section Fumigati by sequence analysis of β-tubulin, calmodulin and actin genes (Samson et al. 2007; Balajee et al. 2005b). ITS sequencing is not recommended.Antifungal susceptibility: Aspergillus fischeri (Australian national data); MIC µg/mL, *MEC µg/mL No <0.03 0.06 0.125 0.25 0.5 1 2 4 8 ≥16 AmB 11 2 5 3 1 ISAV 1 1 VORI 11 1 2 3 4 1 POSA 11 1 4 2 2 2 ITRA 11 2 3 3 3 ANID* 2 2 MICA* 2 2 -
Aspergillus flavus complex
Aspergillus section Flavi historically includes species with conidial heads in shades of yellow-green to brown and dark sclerotia. Hedayati et al. (2007) reviewed the A. flavus complex and included 23 species or varieties, including two sexual species, Petromyces alliaceus and P. albertensis. Several species of section Flavi produce aflatoxins, among which aflatoxin B1 is the most toxic of the many naturally occurring secondary metabolites produced by fungi. Aflatoxins are mainly produced by A. flavus and A. parasiticus, which coexist and grow on almost any crop or food (Varga et al. 2011). Within the complex, A. flavus is the principle medically important pathogen of both humans and animals. However, some other species in the A. flavus complex, notably A. oryzae, A. avenaceus, A. tamari, A. alliaceus and A. nomius, may cause rare mostly superficial infections (Hedayati et al. 2007, de Hoog et al. 2015).
Note: Accurate species identification within A. flavus complex remains difficult due to overlapping morphological and biochemical characteristics. For morphological identifications, it is recommended to report as Aspergillus flavus complex.
Molecular identification: ITS sequence analysis is sufficient to identify to species complex level only. Definitive identification requires analysis of β-tubulin, calmodulin and actin genes (Samson et al. 2007, Balajee et al. 2005a).
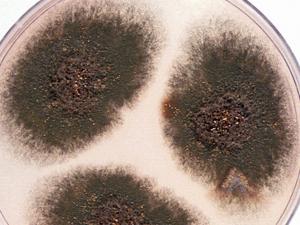
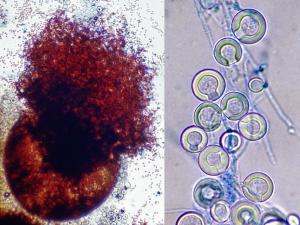
Aspergillus flavus
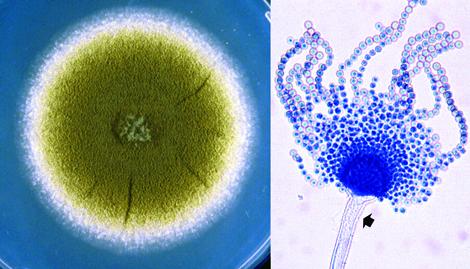
Culture and conidial head of Aspergillus flavus.
Aspergillus flavus has a worldwide distribution and normally occurs as a saprophyte in soil and on many kinds of decaying organic matter, however, it is also a recognised pathogen of humans and animals. It is a causative agent of otitis, keratitis, acute and chronic invasive sinusitis, and pulmonary and systemic infections in immunocompromised patients. A. flavus is second only to A. fumigatus as the cause of human invasive aspergillosis (Hedayati et al. 2007). Although, A. flavus is the principle medically important pathogen of both humans and animals, some other species in the A. flavus complex notably A. oryzae, A. avenaceus, A. tamari, A. alliaceus and A. nomius may cause rare mostly superficial infections (Hedayati et al., 2007; de Hoog et al., 2020).
RG-2 organism.
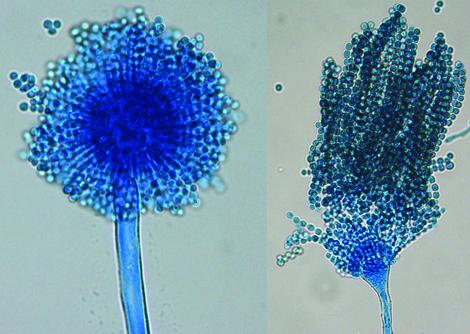
Conidial heads of Aspergillus flavus. Note: conidial heads with both uniseriate and biseriate arrangement of phialides may be present.
Morphological description: On Czapek Dox agar, colonies are granular, flat, often with radial grooves, yellow at first but quickly becoming bright to dark yellow-green with age. Conidial heads are typically radiate, later splitting to form loose columns (mostly 300-400 µm in diameter), biseriate but having some heads with phialides borne directly on the vesicle (uniseriate). Conidiophore stipes are hyaline and coarsely roughened, often more noticeable near the vesicle. Conidia are globose to subglobose (3-6 µm in diameter), pale green and conspicuously echinulate. Some strains produce brownish sclerotia.
Key features: Spreading yellow-green colonies, rough-walled stipes, mature vesicles bearing phialides over their entire surface and conspicuously echinulate conidia.
Antifungal susceptibility: Aspergillus flavus complex (Pfaller et al. 2013a, Australian national data); MIC µg/mL, *MEC µg/mL. No ≤0.016 0.03 0.06 0.125 0.25 0.5 1 2 4 ≥8 AmB 225 1 5 7 42 110 57 3 ISAV 60 7 26 15 9 1 2 VORI 224 1 1 7 51 116 44 2 2 POSA 213 2 5 20 43 109 30 4 ITRA 225 3 13 30 133 42 3 1 ANID* 146 110 24 1 1 1 9 MICA* 146 90 46 1 9 -
Aspergillus fumigatus complex
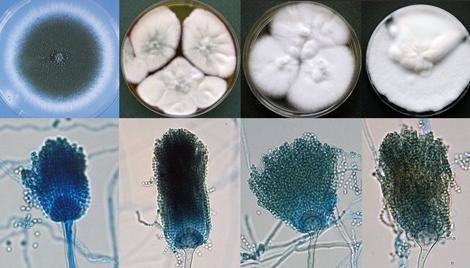
Four species in the Aspergillus fumigatus complex showing overlapping morphological characteristics; (a) Aspergillus fumigatus, (b) Aspergillus lentulus, (c) Neosartorya fischeri and (d) Aspergillus felis.
Aspergillus section Fumigati includes species characterised by uniseriate aspergilla with columnar conidial heads in shades of blue-green and flask-shaped vesicles (Raper and Fennell, 1965). Teleomorphic species belonging to the “Aspergillus fischeri series” of the A. fumigatus group (Raper and Fennell, 1965) were placed in the genus Neosartorya (family Trichocomaceae) by Malloch and Cain (1972). These have now been transferred to Aspergillus and the section Fumigati now includes more than 60 species (Samson et al. 2007).
Although A. fumigatus is recognised as the major human pathogen within the complex, recent phylogenetic studies have demonstrated that some human and animal infections may be caused by A. lentulus, A. fumigatiaffinis, A. fumisynnematus, A. felis, Neosartorya fischeri, N. pseudofischeri, N. udagawae, N. hiratsukae and N. spinsosa (Coriglione et al. 1990; Summerbell et al. 1992; Padhye et al. 1994a; Lonial et al. 1997; Jarv et al. 2004; Balajee et al. 2005, 2006; Barrs et al. 2013).
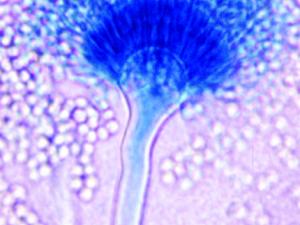
Aspergillus fumigatus
RG-2 organism.
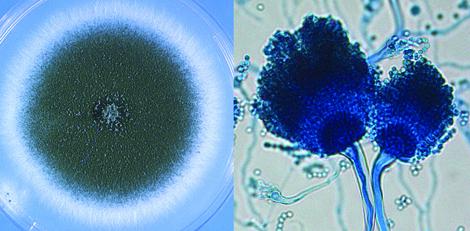
Culture and conidial heads of Aspergillus fumigatus.
Morphological description:
On Czapek Dox agar, colonies are typically blue-green with a suede-like surface consisting of a dense felt of conidiophores. Conidial heads are typically columnar (up to 400 x 50 µm but often much shorter and smaller) and uniseriate. Conidiophore stipes are short, smooth-walled and have conical-shaped terminal vesicles which support a single row of phialides on the upper two thirds of the vesicle. Conidia are produced in basipetal succession forming long chains and are globose to subglobose (2.5-3.0 µm in diameter), green and finely roughened. Note: This species is thermotolerant with a maximum growth temperature of 55C.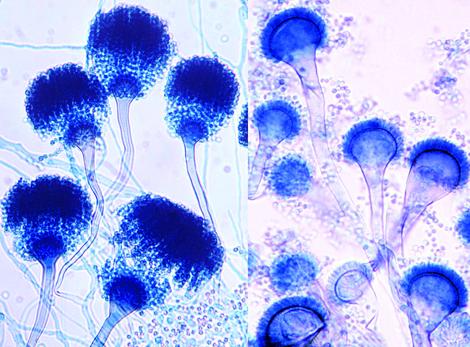
Conidial heads of A. fumigatus. Note: uniseriate row of phialides on the upper two thirds of the vesicle.
Key features:
Uniseriate and columnar conidial heads with the phialides limited to the upper two thirds of the vesicle and curving to be roughly parallel to each other.Molecular identification:
Sequence analysis of ITS is sufficient to identify to species complex level only. For definitive identification analysis, β-tubulin, calmodulin and actin genes is required (Samson et al. 2007; Balajee et al. 2005)Antifungal susceptibility: Aspergullus fumigatus complex (Pfaller et al. 2013a, Australian national data); MIC µg/mL, *MEC µg/mL. No ≤0.016 0.03 0.06 0.12 0.25 0.5 1 2 4 8 16 ≥64 AmB 1367 2 40 123 181 319 609 92 1 ISAV 504 1 2 10 77 277 101 27 3 6 VORI 1331 1 2 30 144 638 395 62 28 15 16 POSA 1262 51 136 224 497 283 45 17 3 1 5 ITRA 1367 10 22 68 205 658 345 37 6 1 1 14 ANID* 761 616 119 21 2 1 2 MICA* 761 653 89 13 4 2 -
Aspergillus lentulus
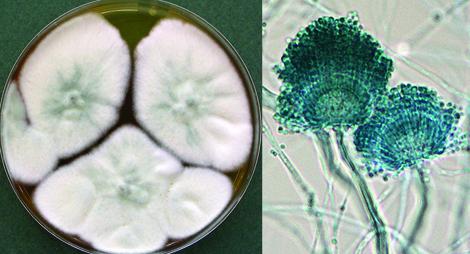
Aspergillus lentulus culture and conidial heads.
Aspergillus lentulus appears to be widely distributed in soil and is now well documented as a causative agent of invasive aspergillosis in immunosuppressed patients. A. lentulus is part of the A. fumigatus complex.
RG-2 organism.
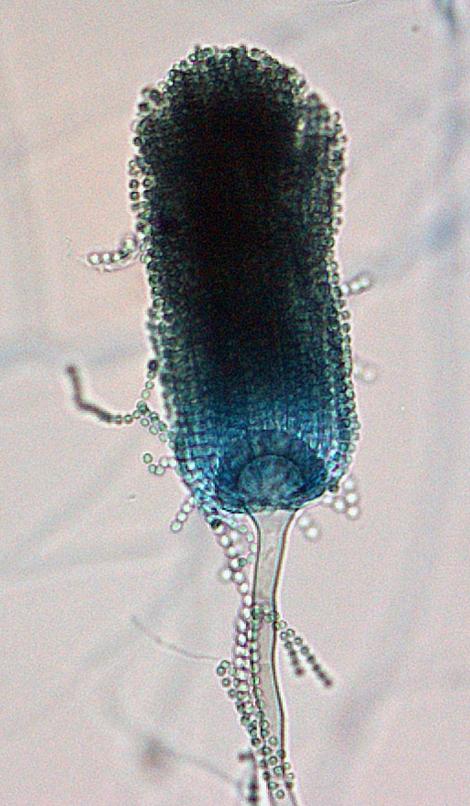
Aspergillus lentulus conidial head morphology.
Morphological description:
Colonies of A. lentulus are suede-like to floccose, white with interspersed grey-green patches of conidia (conidiation is slow to poor in most strains). Conidial heads are short, columnar and uniseriate. Conidiophore stipes are smooth-walled, sometimes sinuous and are often constricted at the neck. Vesicles are usually subglobose in shape. Conidia globose to broadly ellipsoidal (2-3.2 µm in diameter), smooth to finely roughened.Molecular identification:
A. lentulus can be distinguished from other members of the section Fumigati by sequence analysis of β-tubulin, calmodulin and actin genes (Samson et al. 2007, Balajee et al. 2005b). ITS sequencing is not recommended.Antifungal susceptibility: Aspergillus lentulus (Australian national data); MIC µg/mL, *MEC µg/mL No <0.03 0.06 0.125 0.25 0.5 1 2 4 8 ≥16 AmB 22 1 2 2 9 6 2 ISAV 9 1 5 3 VORI 21 4 5 9 3 POSA 20 1 5 4 9 1 ITRA 22 1 5 4 8 2 2 ANID* 9 8 1 MICA* 9 9 -
Aspergillus nidulans complex
Aspergillus subgenus Nidulantes; Gams et al. (1985) includes species with biseriate conidial heads, brown pigmented often short stipes, and green conidia. Cleistothecia are soft-walled, surrounded by Hülle cells, and ascospores are red to purple in colour. Section Nidulantes is one of the largest subgenera of the genus Aspergillus, and includes about 80 species. Several species have been reported as medical pathogens principally Aspergillus nidulans, but also A. sydowii, A. unguis, A. rugulovalvus and A. tetrazonus.
Molecular identification:
ITS sequencing is sufficient to identify to species complex only. A. nidulans can be distinguished from other members of the section Nidulantes by sequence analysis of β-tubulin, calmodulin and actin genes.Aspergillus nidulans
Aspergillus nidulans is a typical soil fungus with a worldwide distribution, it has also been reported to cause disease in human and animals.
RG-1 organism.
Morphological description:
On Czapek Dox agar, colonies are typically plain green in colour with dark red-brown cleistothecia developing within and upon the conidial layer. Reverse may be olive to drab-grey or purple-brown. Conidial heads are short, columnar (up to 70 x 30 µm in diameter) and biseriate. Conidiophore stipes are usually short, brownish and smooth-walled. Conidia are globose (3-3.5 µm in diameter) and rough-walled.Key features:
Conidial heads are short, columnar and biseriate. Stipes are usually short, brownish and smooth-walled. Conidia are globose and rough-walled.Click images below to expand:
Antifungal susceptibility: Aspergillus nidulans complex (Australian national data); MIC µg/mL No ≤0.016 0.03 0.06 0.125 0.25 0.5 1 2 4 ≥8 AmB 67 5 10 5 20 22 5 ISAV 19 5 10 2 1 1 VORI 66 1 3 12 32 13 3 1 1 POSA 63 3 12 13 19 13 1 1 1 ITRA 67 2 6 12 20 24 1 2 ANID* 35 18 10 5 2 MICA* 35 22 11 2 -
Aspergillus niger complex
The black aspergilli, Aspergillus section Nigri (Gams et al. 1985) includes species with uniseriate or biseriate conidial heads, spherical to pyriform vesicles, smooth-walled stipes and black or near black-coloured conidia. This group contains about 26 species with Aspergillus niger being the most common species isolated. A. niger can be isolated from all continents and is not very selective with respect to environmental conditions. Other species within this group that have been linked to human and animal infection include A. acidus, A. aculeatus, A. brasiliensis and A. tubingensis.
Molecular identification:
In Aspergillus section Nigri, all species can be distinguished from each other using calmodulin sequence data, and all except one can be distinguished using β-tubulin sequence data. ITS sequencing can only be used for a rough classification of the uni- and biseriate species (Samson et al. 2007).Aspergillus niger
Aspergillus niger is one of the most common and easily identifiable species of the genus Aspergillus, with its white to yellow mycelial culture surface later bearing black conidia. This species is very commonly found in aspergillomas and is the most frequently encountered agent of otomycosis. It is also a common laboratory contaminant.
RG-1 organism.
Morphological identification:
On Czapek Dox agar, colonies consist of a compact white or yellow basal felt covered by a dense layer of dark-brown to black conidial heads. Conidial heads are large (up to 3 mm by 15 to 20 µm in diameter), globose, dark brown, becoming radiate and tending to split into several loose columns with age. Conidiophore stipes are smooth-walled, hyaline or turning dark towards the vesicle. Conidial heads are biseriate with the phialides borne on brown, often septate metulae. Conidia are globose to subglobose (3.5-5 µm in diameter), dark brown to black and rough-walled.Key features:
Conidial heads are dark brown to black, radiate and biseriate with metulae twice as long as the phialides. Conidia brown and rough-walled.Click images below to expand:
Antifungal susceptibility: Aspergillus niger complex (Pfaller et al. 2013, Australian national data); MIC µg/mL, *MEC µg/mL. No ≤0.016 0.03 0.06 0.125 0.25 0.5 1 2 4 8 ≥16 AmB 229 16 31 35 87 50 10 ISAV 75 1 3 1 10 27 28 3 2 VORI 187 4 9 10 25 81 54 3 1 POSA 223 3 12 39 38 76 53 2 ITRA 229 1 1 6 23 38 104 51 3 1 1 ANID* 128 89 24 6 2 1 MICA* 128 58 45 18 2 5 -
Aspergillus terreus complex
Aspergillus section Terrei (Gams et al. 1985); Aspergillus terreus complex includes species with biseriate, columnar conidial heads in shades of buff to brown (Raper and Fennell 1965). The most important species of this section is A. terreus, which is ubiquitous in the environment (Samson et al. 2011). Two other species have been reported as medical pathogens, A. alabamensis and A. niveus.
Molecular identification: A. terreus can be distinguished from other members of the section Terrei by sequence analysis of β-tubulin, calmodulin and actin genes. ITS sequencing is sufficient to identify to species complex level only.
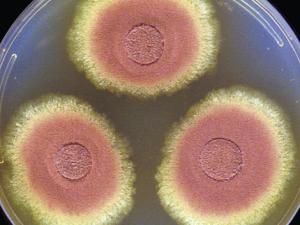
Aspergillus terreus
Aspergillus terreus occurs commonly in soil and is occasionally reported as a pathogen of humans and animals.
RG-2 organism.
Morphological identification:
On Czapek Dox agar, colonies are typically suede-like and cinnamon-buff to sand-brown in colour with a yellow to deep dirty brown reverse. Conidial heads are compact, columnar (up to 500 x 30-50 µm in diameter) and biseriate. Metulae are as long as the phialides. Conidiophore stipes are hyaline and smooth-walled. Conidia are globose to ellipsoidal (1.5-2.5 µm in diameter), hyaline to slightly yellow and smooth-walled.Key features:
Cinnamon-brown cultures, conidial heads biseriate with metulae as long as the phialides.References:
Raper and Fennell (1965), Domsch et al. (1980), McGinnis (1980), Onions et al. (1981), Samson and Pitt (1990), Samson et al. (1995), de Hoog et al. (2000) and Klich (2002).Click images below to expand:
Antifungal susceptibility: Aspergillus terreus complex (Pfaller et al. 2013, Australian national data); MIC µg/mL, *MEC µg/mL. No ≤0.016 0.03 0.06 0.125 0.25 0.5 1 2 4 8 ≥16 AmB 206 1 3 11 37 94 56 4 ISAV 68 1 11 28 20 7 1 VORI 204 5 27 98 57 12 4 1 POSA 194 5 18 36 82 39 9 5 ITRA 207 8 5 24 66 91 11 2 ANID* 128 89 24 6 2 1 MICA* 128 58 45 8 2 5