Infant Formula Use and Decision Making Study (InFormD): Infant formula preparation
Background
Infant formula products are specially formulated to meet the nutritional needs of a vulnerable population. By three-months of age, around 60% of Australian and New Zealand infants will have received some infant formula. Importantly, powdered infant formula is not a sterile product; pathogens may be present in the powder and contamination with bacteria present naturally in the environment can also occur during preparation and storage. Pathogenic risks can, however, be minimised by following the recommended preparation and storage instructions. Caregivers’ ability to understand and follow “on-package” preparation and storage instructions is therefore of high importance.
Project objectives
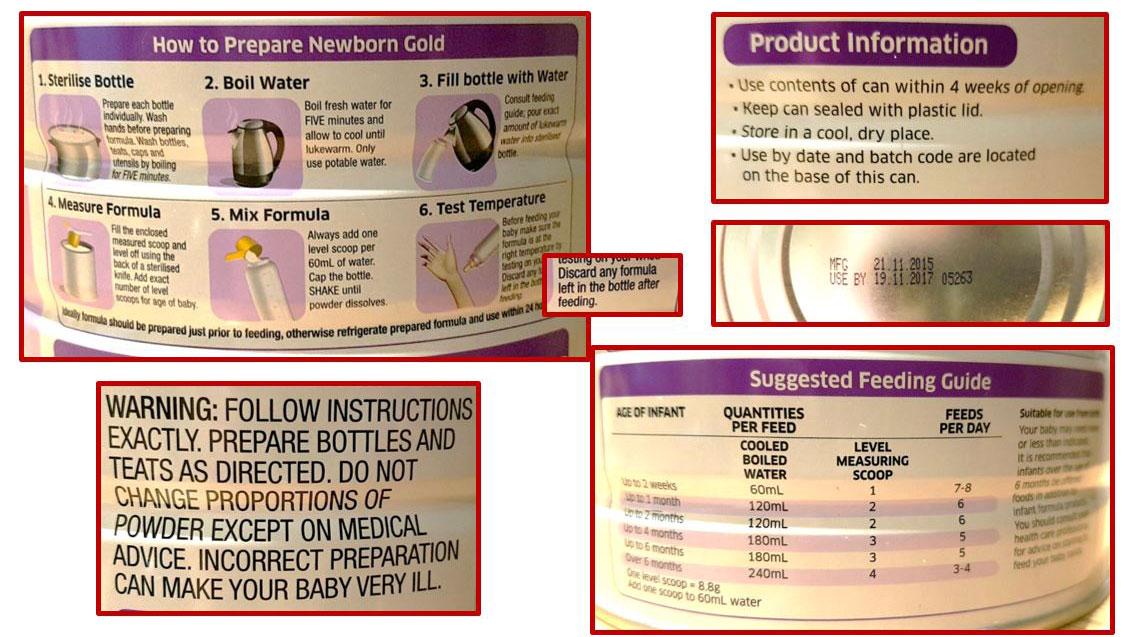
The study aims to increase understanding of how Australian caregivers perceive, interpret and use the following mandatory and voluntary “on package” labelling information on infant formula products when preparing and storing infant formula: step-by-step preparation instructions, instruction to discard unfinished feeds, warning advice on powdered infant formula, storage instructions for the package of formula, use-by/best-before date.
The findings from this study will help to inform Food Standards Australia New Zealand’s review of infant formula product regulations in the Australia New Zealand Food Standards Code.
Objective: To determine how consumers use and understand the following information when preparing infant formula:
Part 2: Infant formula preparation & storage
Aim: To explore how Australian caregivers use and understand labelling information on infant formula products when preparing infant formula at home.
- 30 caregivers living in metropolitan Adelaide
- Infants aged 0-12 months
- Prepare at least 50% of formula feeds
- Home visits
- Eye-tracking task
- Retrospective think aloud interview with eye gaze replay
- In-depth interview
Project outputs
Researchers involved in this project are preparing a paper with the key findings. The abstract is below.
Abstract: Infant formula products are specially formulated to meet the nutritional needs of a vulnerable population. Caregivers’ ability to understand and follow preparation and storage instructions is therefore of high importance. This study aims to increase understanding of how Australian caregivers perceive, interpret and use mandatory and voluntary “on-package” labelling information when preparing and storing infant formula. An eye-tracking task requiring caregivers (n=30) to prepare an unfamiliar infant formula product while wearing Tobi Pro 2 Glasses revealed that almost all caregivers look at the preparation instructions (93%) and feeding guide (87%); fewer look at the warning advice (43%) and storage instructions (27%); and none look at the date-marking. The same trend was observed with respect to fixation duration. Findings from retrospective think-aloud and in-depth interviews conducted immediately after the eye-tracking task, revealed that while the instructions are generally understood, they are not always adhered to, with most caregivers making modifications for efficiency or convenience. Lack of awareness and low perceived risk to the infant’s health were other reasons for non-adherence. These findings suggest that mandated food-safety elements on infant formula products need to be clearer, more comprehensible and more effective, to ensure safe preparation and storage by all caregivers.
Project partners
Collaborating institutions:
Food Standards Australia New Zealand
Funding:
Food Standards Australia New Zealand (January-June 2016)
Contact
GFAR researchers involved in this project:
